Nosotros no hemos recibido el espíritu del mundo, sino el Espíritu que procede de Dios (1 Cor 2, 12), el Espíritu de su Hijo, que Dios envió a nuestros corazones (Gal 4,6). Y por eso predicamos a Cristo crucificado, escándalo para los judíos y locura para los gentiles, pero para los llamados, tanto judíos como griegos, es Cristo fuerza de Dios y sabiduría de Dios (1 Cor 1,23-24). De modo que si alguien os anuncia un evangelio distinto del que recibisteis, ¡sea anatema! (Gal 1,9).
Páginas
- EL RINCÓN CATÓLICO (Aprendamos Latín)
- Latín padre Francisco Torres
- Historia sacra en latín
- CURSO BÍBLICO
- TOMÁS DE AQUINO
- SUMA DE TEOLOGÍA
- FILOSOFÍA Y LITERATURA
- HISTORIA
- CONSERVANDO LA FE
- LA SACRISTÍA DE LA VENDÉE
- P. ALFONSO GÁLVEZ
- P. JAVIER OLIVERA
- P. SANTIAGO MARTÍN
- AGENDA 2030
- FIDUCIA SUPPLICANS
- EL TORO TV
- EL ROSARIO EN FERRAZ , por José Andrés Calderón
- TEOLOGÍA DEL CUERPO DEL PAPA JUAN PABLO II ... Y RELACIONADOS
- CÓNCLAVE INFORMA
- EN LA IGLESIA
- LEÓN XIV
- CATECISMO
BIENVENIDO A ESTE BLOG, QUIENQUIERA QUE SEAS
viernes, 14 de octubre de 2022
Una directiva de Pfizer reconoce que no sabían si su vacuna era efectiva contra el COVID
domingo, 16 de enero de 2022
El Papa Francisco y los pobres de ‘Pfizer’, en el Vaticano la forma es sustancia, el ‘Chrislam’, América deja de ser católica.
sábado, 1 de enero de 2022
Nos hemos quedado sin palabras cuando hemos visto el último hallazgo realizado por La Quinta Columna en el potingue que es la "vacuna" de Pfizer (Ricardo Delgado)
Aquí se detallan pruebas contundentes e incontestables de lo que realmente se está inoculando con las "vacunas" Pfizer en el organismo de las personas. Se trata de un auténtico genocidio que hay que denunciar en masa. El primero en hacerlo va a ser Ricardo Delgado Ruiz de la Quinta Columna. Este video debe de ser conocido por todo el mundo mundial para atajar lo más pronto posible esta inoculación de lo que no es sino un tóxico con repercusiones muy graves. Ya está bien de cerrar los ojos a la realidad. No hay aquí ningún negacionismo ni nada de tipo conspiranoico. Son pruebas experimentales del contenido real de estas "vacunas". Si no se ve es porque no se quiere ver, pero es algo que pone los pelos de punta, lo que están haciendo con la gente, abusando de su ignorancia.
sábado, 11 de diciembre de 2021
La extraña "revolución" contenida en las vacunas Pfizer y Moderna ARNm
Las vacunas Pfizer y Moderna son en realidad formas de "terapia génica", ya que se basan en la administración de un ácido nucleico - ARN mensajero- que, una vez que ha penetrado en las células humanas, transfiere una sola información: la necesaria para producir grandes cantidades de proteína Spike que, una vez reconocidas como "extrañas", activarán la correspondiente producción de anticuerpos. Por tanto, es un "tratamiento" destinado a modificar la información y la actividad genética de la célula.
El objetivo es claro: suplantar el aparato farmacológico tradicional con medicamentos de ARN (o ADN) construidos por computadora (basados en secuencias). Esta deriva es peligrosa, no solo porque pretende revolucionar el enfoque farmacológico clásico en ausencia de fundamentos científicos válidos, sino porque acabaría concentrando la posibilidad de producir fármacos en manos de unas pocas multinacionales.
domingo, 15 de agosto de 2021
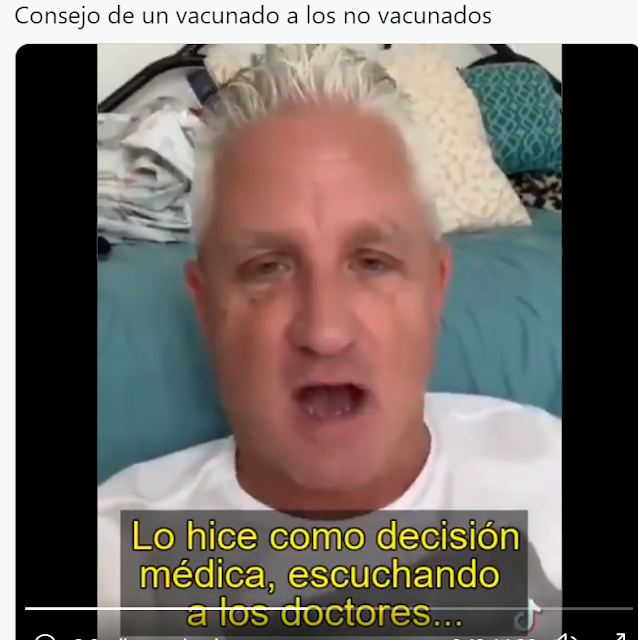
Consejo de un vacunado a los no vacunados: “Manténganse firmes en su postura, yo desearía no haberme vacunado nunca”
VIDEO
Duración 1:26 minutos
https://twitter.com/i/status/1426450297887592451
martes, 3 de agosto de 2021
miércoles, 23 de junio de 2021
Los miserables de la OMS modifican el artículo en el que decían que los “niños no deben vacunarse” y ahora ponen “la vacuna de Pfizer es adecuada para su uso por personas de 12 años o más”


Este oscurantismo, esta manipulación, este descaro demuestra que el organismo que debería ocuparse y preocuparse de la salud de toda la humanidad está dirigido por criminales a los que no les importa lo que suframos todos, niños incluidos, con tal de seguir llenándose sus asquerosos bolsillos. Esto es de lo más grave que hemos visto en todo este año y medio que llevamos de mentiras y de manipulación.
jueves, 18 de febrero de 2021
Vaticano del Covid-19: los que se negaron a ser vacunados pueden ser despedidos
sábado, 23 de enero de 2021
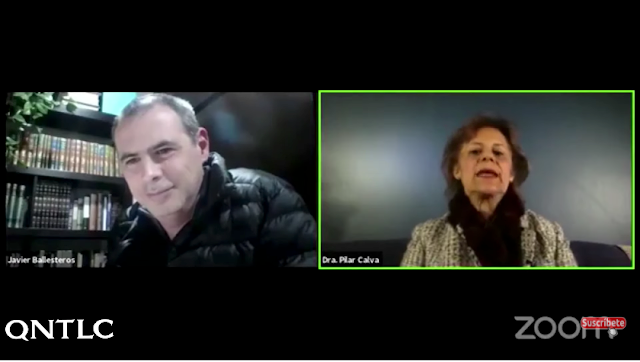
Pregunta a la doctora María Pilar Calva Mercado sobre qué vacunas proceden de fetos abortados
Este video está tomado de una conferencia magistral de la Dra María Pilar Calva Mercado, médica genetista y discípula del venerable Dr. Lejeune. Su título es "Juicio ético y genético de las vacunas contra el Covid-19" cuya duración es 1:31:12
Padre Javier Olivera Ravasi
martes, 19 de enero de 2021
Vacunas del Covid: Parroquias católicas son los “principales jugadores”
martes, 12 de enero de 2021
NOTICIAS VARIAS 12 de enero de 2021
MENTE ALTERNATIVA
REACCIONES ADVERSAS INFORMADAS DE LA VACUNACIÓN CONTRA EL COVID19 (REFERENCIAS ADJUNTAS)
THIERRY MEYSSAN: BIDEN Y EL PODER POR LA FUERZA
EL INVESTIGADOR.ORG
Listado de plataformas de comunicación resistentes a la censura, que respetan la libertad de expresión y tu privacidad
INFOVATICANA
La radio de los obispos considera ‘buena noticia’ el encumbramiento de una enemiga de la fe (Carlos Esteban)
El Vaticano modifica el rito de imposición de la ceniza durante la pandemia (Carlos Esteban)
GLORIA TV
Cardenal Sarah ordena Rito de Miércoles de Cenizas para el Covid-19
viernes, 8 de enero de 2021
jueves, 7 de enero de 2021
Update: COVID-19 Vaccine Candidates and Abortion-Derived Cell Lines
El enlace a la página web es éste:
To view this chart as a PDF, see: COVID-19 Vaccine Candidates and Abortion-Derived Cell Lines
Updated January 4, 2021
Accurate information about the development and production of COVID-19 vaccines is essential, especially because many proposed candidates use newer molecular technologies for production of a viral vaccine. One concern regarding the ethical assessment of viral vaccine candidates is the potential use of abortion-derived cell lines in the development, production or testing of a vaccine. This analysis utilizes data from the primary scientific literature when available, along with data from clinical trial documents, reputable vaccine tracking websites, and published commercial information.1 It is the hope that by providing accurate data, recipients can make well-informed decisions regarding vaccine choices.
For additional background and guidance, please see:
* A Visual Aid to Viral Infection and Vaccine Production for a visual primer on the various strategies for viral vaccine production.
* COVID-19 Vaccines & Fetal Cell Lines for an infographic description of how fetal cell lines are sometimes used to produce vaccines.
Flow Chart for Creation and Testing of Vaccines
 | Design & Development: conceptualization, preparatory experiments, and specification for how vaccine will be constructed and produced. Production: process used to manufacture final vaccine to be given to people.
Confirmatory Lab Tests on Product: tests to analyze quality, nucleic acid or protein sequence, protein conformation, antibody reactivity, etc. of final vaccine product.
Vaccination: giving final produced vaccine to people. | ||||||
NOTA: La tabla que se proporciona, a continuación, se entenderá mejor escuchando de nuevo los minutos 26 a 30 aproximadamente, de la entrevista a la dra. Pilar Calva que llevó a cabo el padre Javier Olivera Ravasi.
🟩
Recombinant DNA sequence in Agrobacterium, transformation of plant cells
🟩
Plant expression of protein and VLP
🟩🔺
Pseudovirus
HEK293 cells
| Analysis of SARS-CoV-2 (COVID-19) Vaccine Candidates Last Updated 4 January 2020
| 🟩 DOES NOT USE abortion-derived cell line
🔺 DOES USE abortion-derived cell line
🟩🔺 SOME tests DO NOT use abortion-derived cells, SOME DO.
� Currently undetermined | ||||||
| Sponsor(s)1 | Country | Strategy2 | Clinical Trial Status3 | Public Funding4 | Design & Development | Production | Confirm-atory Lab Tests |
| WHOLE VIRUS VACCINE – LIVE ATTENUATED or INACTIVATED | |||||||
| Beijing Institute of Biological Products/ Sinopharm | China | Inactivated virus “BBIBP-CorV” Given: Intramuscular 2 doses (2 weeks apart) | Early approval in China | 🟩 Vero monkey cells
| 🟩 Vero monkey cells
| 🟩 Cytopathic test | |
| Wuhan Institute of Biological Products/ Sinopharm | China | Inactivated virus Unnamed Given: Intramuscular 2 doses (2 weeks apart) | Phase 3 Early approval in China | 🟩 Vero monkey cells | 🟩 Vero monkey cells | 🟩 Plaque reduction neutralization test Vero monkey cells Xia et al., JAMA 324, 951, 13Aug2020 | |
| Bharat Biotech/Indian Council of Medical Research | India | Inactivated virus “BBV152” Given: Intramuscular2 doses (2 weeks apart) | India EUA granted | 🟩 Vero monkey cells | 🟩 Vero monkey cells | 🟩 Antibody ELISA Plaque reduction Vero monkey cellsYadav et al., ResearchSquare 10Sept2020 | |
| John Paul II Medical Research Institute | USA | Live attenuated virus
| Pre-clinical | 🟩 | 🟩 | � | |
| Sinovac Biotech Co., Ltd. | China | Inactivated virus “PiCoVacc” Given: Intramuscular 2 doses (2 weeks apart) | Phase 3 Early approval in China | 🟩 Vero monkey cells
| 🟩 Vero monkey cells | 🟩🔺 protein test HEK293 cells | |
| Valneva and Dynavax | France USA UK | Inactivated Virus “VLA2001” plus adjuvant CpG1018 Given: Intramuscular | Pre-clinical | 🟩 Vero monkey cells
| 🟩 Vero monkey cells | � | |
| VIRAL VECTOR-BASED VACCINE | |||||||
| Altimmune | USA | Replication-deficient Adenovirus vector “AdCOVID” Given: Intranasal | Pre-clinical | 🔺 PER.C6 cells | 🔺 PER.C6 cells | 🔺 | |
| AstraZeneca University of Oxford
| USA UK | Replication-deficient Adenovirus vector “AZD1222” “ChAdOX1nCoV-19” Given: Intramuscular 2 doses (4 weeks apart) | UK EUA granted India EUA grantedPhase 3 | Operation Warp Speed HHS-BARDA $1.2 Billion CEPI up to $384 Million | 🔺 HEK293 cells | 🔺 HEK293 cells | 🔺 |
| CanSino Biologics, Inc. Beijing Institute of Biotechnology, Academy of Military Medical Sciences, PLA of China | China | Replication-deficient Adenovirus vector “Ad5-nCoV” Given: Intramuscular 1 dose | Phase 3 | 🔺 HEK293 cells | 🔺 HEK293 cells | 🔺 | |
| Gamaleya Research Institute | Russia | Replication-deficient Adenovirus vectors (rAd26-S+rAd5-S) “Sputnik V” Given: Intramuscular 2 doses (3 weeks apart) | Phase 3 Early approval in Russia August 2020 | 🔺 HEK293 cells | 🔺 HEK293 cells | 🔺 | |
| ImmunityBio and NantKwest | USA | Replication-deficient Adenovirus vector recombinant “hAd5 S-Fusion + N-ETSD” Given: Subcutaneous | Phase 1 | 🔺 E.C7 cells (derivative of HEK293 cells) Rice et al., bioRxiv 30July2020 | 🔺 E.C7 cells | 🔺 Protein and antibody tests HEK293T cells Rice et al., bioRxiv 30July2020 Seiling et al., medRxiv 6Nov2020 | |
| Institut Pasteur and Themis and Merck | USA France | Replication-competent recombinant measles virus “TMV-083” Given: Intramuscular | Phase 1/2 | CEPI up to $4.9 Million | 🔺HEK293T Development and rescue of recombinant measles virus “SARS-CoV-2 S-encoding vaccine candidates… were generated as described previously” | 🟩 Vero monkey cells | 🟩🔺 Lentiviral vectors for antigenic DC Fusogenic test HEK293T Fusogenic test S protein expression Vero monkey cells |
| Israel Institute for Biological Research (IIBR) | Israel | Replication-competent recombinant vesicular stomatitis virus (VSVΔG) “IIBR-100” Given: Intramuscular1 dose | Phase 1 | 🟩 BHK hamster cells Vero monkey cells Yahalom-Ronen et al., bioRxiv 19June2020 | 🟩 Vero monkey cells Yahalom-Ronen et al., bioRxiv 19June2020 | 🟩 Plaque reduction; immunofluorescence Vero monkey cells Yahalom-Ronen et al., bioRxiv 19June2020 | |
| Janssen Research & Development, Inc. Johnson & Johnson | USA | Replication-deficient Adenovirus vector “Ad26” 1 or 2 doses (8 weeks apart) | Phase 3 | Operation Warp Speed HHS-BARDA $1,457,887,081 total | 🔺 PER.C6 cells | 🔺 PER.C6 cells | 🔺 |
| Merck and IAVI | USA | Replication-competent recombinant vesicular stomatitis virus (VSVΔG) “V590” Given: Intramuscular | Pre-clinical | Operation Warp Speed HHS-BARDA $38,033,570 | 🟩 Vero monkey cells | 🟩 Vero monkey cells | � |
| Shenzhen Geno-immune Medical Institute | China | Lentivirus minigenes + Adult human APC (antigen-presenting cells)
| Phase 1 | � | 🟩 | � | |
| Shenzhen Geno-immune Medical Institute | China | Lentivirus minigenes + Adult human CD/T cells (dendritic cells and T cells) “LV-SMENP-DC” | Phase 1/2 | � | 🟩 | � | |
| Vaxart | USA | Replication-deficient Adenovirus vector “VXA-CoV2-1” plus dsRNA adjuvant Given: Oral | Phase 1 | 🔺 HEK293 cells | 🔺 HEK293 cells | 🔺 | |
| PROTEIN-BASED VACCINE | |||||||
| Anhui Zhifei Longcom Biopharmaceutical/Institute of Microbiology, Chinese Academy of Sciences | China | Protein vaccine Recombinant RBD dimer plus adjuvant Given: Intramuscular 2 or 3 doses (30 days apart) | Phase 3 | 🔺 HEK293T cells | 🟩 CHO hamster cells | 🔺 Pseudovirus HEK293T cells | |
| Clover Biopharmaceuticals, Inc. | China | Protein vaccine “SCB-2019” plus adjuvant CpG 1018 Given: Intramuscular | Phase 1 | CEPI up to $69.5 Million | 🟩cDNA in expression vector; transfect CHO hamster cells | 🟩 CHO hamster cells | 🟩🔺Pseudovirus HEK293 cells Ref’d: Nie et al., Emerging Microbes & Infections 24Mar2020 Cytopathic effect Vero monkey cells |
| Federal Budgetary Research Institution State Research Center of Virology and Biotechnology “Vektor” | Russia | Protein vaccine “EpiVacCorona” chemically synthesized peptide antigens of SARS-CoV-2, conjugated to a carrier protein adsorbed on an aluminum-containing adjuvant Given: Intramuscular2 doses (3 weeks apart) | Early approval in Russia Oct 2020 | � | 🟩 chemically synthesized peptide antigens | � | |
| John Paul II Medical Research Institute | USA | Recombinant Protein Perinatal human cells (term umbilical cord and placental) | Pre-clinical | 🟩 | 🟩 | � | |
| Kentucky BioProcessing, Inc. (British American Tobacco) | USA | Protein vaccine “KBP-201” Plant-expressed RBD Given: Intramuscular2 doses (3 weeks apart) | Phase 1/2 | 🟩 Recombinant DNA sequence for RBD of SARS-CoV-2 | 🟩 Plant expression of RBD peptide | � | |
| Medicago | Canada | Protein on Virus-Like Particle “CoVLP” Plant-expressed spike protein particle with adjuvant, CpG1018 or AS03 Given: Intramuscular 2 doses (3 weeks apart) | Phase 2/3 | ||||
| Novavax | USA | Protein vaccine “NVX-CoV2373” Baculovirus expression plus Matrix M adjuvant Given: Intramuscular 2 doses (3 weeks apart) | Phase 3 | Operation Warp Speed HHS-BARDA $1,600,434,523 CEPI up to $388 Million | 🟩 | 🟩 Sf9 insect cells | 🟩🔺 Pseudovirus HEK293 cells |
| Sanofi and GSK Protein Sciences
| USA France | Protein vaccine Baculovirus expression plus AS03 adjuvant Given: Intramuscular 2 doses (3 weeks apart) | Phase 1/2 | Operation Warp Speed HHS-BARDA $2,072,775,336 total | 🟩 | 🟩 Sf9 insect cells Baculovirus expressed recombinant protein ;
| � |
| Sorrento | USA | Protein vaccine “T-VIVA-19” SARS-Cov-2 spike protein S1 domain fused with human IgG-Fc Given: Intramuscular | Pre-clinical | 🟩 | 🟩 CHO cells | 🟩 Antibody ELISA; Neutralization assays Vero monkey cells | |
| Sorrento | USA | Protein vaccine “STI-6991” SARS-Cov-2 spike protein expressed on K562 cells | Pre-clinical | � | 🟩 K562 cells | � | |
| University of Pittsburgh | USA | Protein vaccine Adenovirus-expressed recombinant proteins “PittCoVacc” Given: Microneedle arrays | Pre-clinical | 🔺 HEK293 cells | 🔺 HEK293 cells | 🔺 | |
| University of Queensland and CSL Ltd. | Australia | Protein vaccine “V451” Recombinant protein with proprietary molecular clamp Given: Intramuscular | HALTED | CEPI up to $4.5 Million | 🟩 | 🟩 expiCHO hamster cells
| � |
| RNA VACCINE | |||||||
| Arcturus Therapeutics | USA | mRNA vaccine self-transcribing, replicating “LUNAR-CoV19” (“ARCT-021”) in vitro transcription reaction with T7 RNA polymerase from STARR plasmid template LUNAR proprietary lipid nanoparticle encapsulated Given: Intramuscular 1 dose | Phase 2 | 🟩 Sequence designed on computer | 🟩 No cells used | 🟩🔺 protein test HEK293 | |
| CureVac | Germany | mRNA vaccine non-replicating “CVnCoV” in vitro transcription lipid nanoparticle encapsulated Given: Intramuscular 2 doses (4 weeks apart) | Phase 2/3 | CEPI up to $15.3 Million | 🟩 Sequence designed on computer | 🟩 No cells used | 🟩 Protein test Reticulocyte lysate, |
| Moderna, Inc. with National Institutes of Health | USA | mRNA vaccine non-replicating “mRNA-1273” T7 RNA polymerase-mediated transcription from DNA plasmid template LNP (lipid nanoparticle) encapsulated Given: Intramuscular 2 doses (4 weeks apart) | FDA Emergency Use Authorization Approved | Operation Warp Speed HHS-BARDA $2,479,894,979 total CEPI up to $1 Million | 🟩 Sequence designed on computer | 🟩 No cells used | 🟩🔺 protein test & pseudovirus HEK293 cells |
| Pfizer and BioNTech | USA Germany | mRNA vaccine non-replicating “BNT-162a1,b1,b2,b3,c2” nucleoside-modified mRNA in vitro transcribed by T7 polymerase from a plasmid DNA template LNP (lipid nanoparticle) encapsulated Given: Intramuscular 2 doses (3 weeks apart) | FDA Emergency Use Authorization Approved UK EUA granted
| Operation Warp Speed HHS-BARDA $1.95 Billion | 🟩 Sequence designed on computer | 🟩 No cells used | 🟩🔺 protein test & pseudovirus HEK293 cells |
| Sanofi Pasteur and Translate Bio | USA France | mRNA vaccine non-replicating “MRT5500” synthesized by in vitro transcription employing RNA polymerase with a plasmid DNA template LNP (lipid nanoparticle) encapsulated Given: Intramuscular | Pre-clinical | 🟩 Sequence designed on computer | 🟩 No cells used | 🟩🔺 protein test & pseudovirus HEK293 cells | |
| DNA VACCINE | |||||||
| Genexine | Korea | DNA vaccine “GX-19”style=”border: 1px solid black;” DNA synthesized in vitro, placed in plasmid vector Given: Intramuscular and Electroporation 2 doses (4 weeks apart) | Phase 1/2 | 🟩 Sequence designed on computer | 🟩 No cells used | � | |
| Inovio Pharmaceuticals | USA | DNA vaccine “INO-4800” DNA synthesized in vitro, placed in plasmid vector Given: Intradermal Electroporation 2 doses (4 weeks apart) | Phase 2/3 | Operation Warp Speed CEPI up to $22.5 Million | 🟩 Sequence designed on computer | 🟩 No cells used | 🟩🔺 protein test & pseudovirus HEK293 cells |
| Symvivo Corporation | Canada | DNA vaccine Genetically engineered Bifidobacterium longum “bacTRL-spike” Given: Oral, bacteria bind to gut lining 1 dose | Phase 1 | � | 🟩 No cells used | � | |
- Data accumulated from primary literature as referenced in the Chart; AND “COVID-19 Treatment and Vaccine Tracker,” Milken Institute, https://covid-19tracker.milkeninstitute.org/ ; AND “Draft landscape of COVID-19 candidate vaccines,” World Health Organization (WHO), https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines
NOTE that patents are not considered because they are unreliable sources; even the most relevant patents are prospective documents that provide examples of potential use, but do not provide information about actual, current application of an invention or technology. - Prentice, DA and Sander Lee, T. June 15, 2020. A Visual Aid to Viral Infection and Vaccine Production. On Science Series 1. Accessed 19 June 2020 at: https://lozierinstitute.org/a-visual-aid-to-viral-infection-and-vaccine-production/
- Phases of Clinical Trials: Pre-clinical- laboratory and animal studies; Phase I- 10-100 people, study safety and dosage; Phase II- tens to hundreds of people, study efficacy, dosage, side effects; Phase III- hundreds to thousands of people, study efficacy and adverse reactions.
- HHS-BARDA = U.S. Health and Human Services-Biomedical Advanced Research and Development Authority; CEPI = Coalition of Epidemic Preparedness Innovations; BARDA’s rapidly-expanding COVID-19 medical countermeasure portfolio. Accessed 29 Sept 2020 at https://www.medicalcountermeasures.gov/app/barda/coronavirus/COVID19.aspx; CEPI’s COVID-19 Vaccine Portfolio, Accessed 29 Sept 2020 at https://cepi.net/COVAX/
--------
Vacunación DESMONTADA por la doctora María José Martínez Albarracín (video 6 minutos)